Merative Clinical DevelopmentFormerly IBM Clinical Development / Watson Health
Overview
What is Merative Clinical Development?
IBM Clinical Development (formerly eClinicalOS) is a cloud-based electronic data capture (EDC) system.
Recent Reviews
Awards
Products that are considered exceptional by their customers based on a variety of criteria win TrustRadius awards. Learn more about the types of TrustRadius awards to make the best purchase decision. More about TrustRadius Awards
Pricing
Entry-level set up fee?
- Setup fee optional
For the latest information on pricing, visithttps://www.ibm.com/products/clinical…
Offerings
- Free Trial
- Free/Freemium Version
- Premium Consulting/Integration Services
Would you like us to let the vendor know that you want pricing?
23 people also want pricing
Alternatives Pricing
Product Details
- About
- Integrations
- Competitors
- Tech Details
- Downloadables
- FAQs
What is Merative Clinical Development?
Merative® Clinical Development (formerly IBM® Clinical Development) is a unified, cloud-based Clinical Data Management System designed to help reduce the cycle time to start, amend, and manage clinical studies — enabling life sciences organizations to deliver therapies and innovations faster to their patients.
Boasting a proven track record of over 3000+ clinical trials, in 90+ countries, with 1 million+ participants, the vendor states Merative® Clinical Development will help pharmaceutical, medical device, and CROs to shorten study cycle time and reduce costs.
In addition to its core electronic data capture (EDC) functionality, Merative® Clinical Development offers a fully-integrated portfolio of modules that are designed to suit every trial, including:
Medical Coding with AI- Designed to increase efficiency by leveraging AI to build consistency and reduce errors
eConsent - Deliver quick and easy remote participant consenting without additional EDC integration
Open Platform Data Integration - Allows users to build and automate data connectors with minimal coding
Reporting and Analytics - Provide Data Managers to use pre-built or custom reports to derive single and cross-study insights
Electronic Clinical Outcome Assessment (eCOA) - Strengthens Participant Centricity with live, automatic data sync with EDC
Visit https://www.merative.com/clinical-development to learn more.
Boasting a proven track record of over 3000+ clinical trials, in 90+ countries, with 1 million+ participants, the vendor states Merative® Clinical Development will help pharmaceutical, medical device, and CROs to shorten study cycle time and reduce costs.
In addition to its core electronic data capture (EDC) functionality, Merative® Clinical Development offers a fully-integrated portfolio of modules that are designed to suit every trial, including:
Medical Coding with AI- Designed to increase efficiency by leveraging AI to build consistency and reduce errors
eConsent - Deliver quick and easy remote participant consenting without additional EDC integration
Open Platform Data Integration - Allows users to build and automate data connectors with minimal coding
Reporting and Analytics - Provide Data Managers to use pre-built or custom reports to derive single and cross-study insights
Electronic Clinical Outcome Assessment (eCOA) - Strengthens Participant Centricity with live, automatic data sync with EDC
Visit https://www.merative.com/clinical-development to learn more.
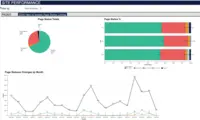
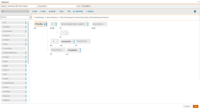
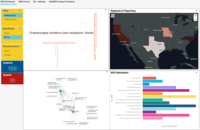
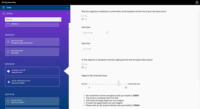
Merative Clinical Development Screenshots
Merative Clinical Development Integrations
- BSI CTMS
- Veeva Clinical Platform
- Argus
- Transperfect
- ALMAC
- Fisher
- Medable
- TRI
- CluePoints
- Catalent
- Optum
Merative Clinical Development Competitors
Merative Clinical Development Technical Details
| Deployment Types | Software as a Service (SaaS), Cloud, or Web-Based |
|---|---|
| Operating Systems | Unspecified |
| Mobile Application | Apple iOS, Android, Windows Phone, Blackberry, Mobile Web |
| Supported Countries | Worldwide usage |
| Supported Languages | 50 + languages and dialects |
Merative Clinical Development Downloadables
Frequently Asked Questions
IBM Clinical Development (formerly eClinicalOS) is a cloud-based electronic data capture (EDC) system.
Medidata Rave EDC, Veeva Vault PromoMats, and Medrio are common alternatives for Merative Clinical Development.
Reviewers rate Support Rating highest, with a score of 8.6.
The most common users of Merative Clinical Development are from Mid-sized Companies (51-1,000 employees).