CaliberLIMS vs. Scifeon ELN & LIMS
CaliberLIMS vs. Scifeon ELN & LIMS
| Product | Rating | Most Used By | Product Summary | Starting Price |
|---|---|---|---|---|
CaliberLIMS | Mid-Size Companies (51-1,000 employees) | CaliberLIMS is an LIMS system designed to meet regulatory compliance requirements. Equipped with multiple modules to choose from, including a built-in ELN and integrated software like Batch Record Management (CaliberBRM), Electronic Logbook ( CalibereLog), Document Management System (Caliber DMS), and Quality Assurance Management System (CaliberQAMS), Caliber LIMS aims to serve as the best laboratory partner on the path to digital transformation. | N/A | |
Scifeon ELN & LIMS | N/A | Scifeon ELN & LIMS is a modular, low-code digital platform designed to manage research and quality-control data across biotech, pharma, and CDMO/CRO environments. It combines Electronic Lab Notebook (ELN) and Laboratory Information Management System (LIMS) capabilities in a single environment, enabling scientists to capture, analyze, and share data. Key Features Integrated ELN & LIMS: Unified data capture, sample management, and process… | $180 per month per user |
| CaliberLIMS | Scifeon ELN & LIMS | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Editions & Modules | No answers on this topic | No answers on this topic | ||||||||||||||
| Offerings |
| |||||||||||||||
| Entry-level Setup Fee | Required | Optional | ||||||||||||||
| Additional Details | — | — | ||||||||||||||
| More Pricing Information | ||||||||||||||||
| CaliberLIMS | Scifeon ELN & LIMS | |
|---|---|---|
| Likelihood to Recommend | 9.0 (1 ratings) | - (0 ratings) |
| CaliberLIMS | Scifeon ELN & LIMS | |
|---|---|---|
| Likelihood to Recommend | Caliber Technologies
| Scifeon No answers on this topic |
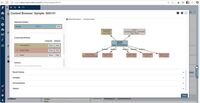
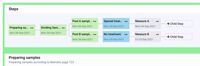
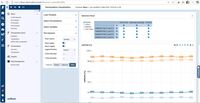
| ScreenShots | CaliberLIMS Screenshots | Scifeon ELN & LIMS Screenshots |