Overview
What is Cloudbyz eTMF?
Cloudbyz eTMF provides a solution that simplifies the complex process of access, retention, and retrieval of clinical trial documents, while ensuring that compliance requirements are fully met.

Leaving a review helps other professionals like you evaluate Document Management Systems
Be the first one in your network to review Cloudbyz eTMF, and make your voice heard!
Get StartedPricing
Entry-level set up fee?
- Setup fee required
Offerings
- Free Trial
- Free/Freemium Version
- Premium Consulting/Integration Services
Would you like us to let the vendor know that you want pricing?
Alternatives Pricing
Product Demos
Cloudbyz eTMF Demo | DIA & Custom Folder Structure with 1-Click! | Archival | Report Subscription
Product Details
- About
- Competitors
- Tech Details
- Downloadables
What is Cloudbyz eTMF?
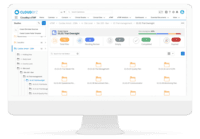
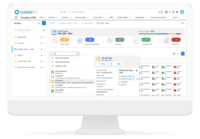
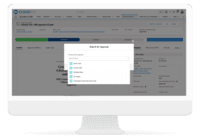
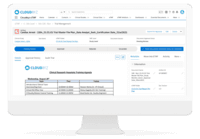
Cloudbyz eTMF solution offers a cloud-based repository of all clinical trial documents including files, images, information, etc. Digitally store, manage, and share all clinical trial-related documents with a centralized overview. Manage essential trial documents, stay inspection ready, and enable real-time visibility for CROs, sponsors, monitors, and other stakeholders in a clinical trial. Cloudbyz eTMF functionality includes-
eTMF Export
Automated & Configurable Metadata
TMF Reference Models
Actionable Notifications
eTMF Locking
Search and Retrieval
eISF Connectivity
Real Time Collaboration
TMF Health Check
Inspection Readiness
- Milestone Tracking
All Cloudbyz solutions are natively built on the Salesforce platform and leverage a common data model to ensure seamless integration between the systems. Additionally, users can leverage the template-based approach to accelerate their trial operations. Their solutions span across the entire life cycle of clinical trials and help customers to stay fully compliant with global industry regulations.
Cloudbyz eTMF Features
- Supported: eTMF Export
- Supported: Automated & Configurable Metadata
- Supported: TMF Reference Models
- Supported: Actionable Notifications
- Supported: eTMF Locking
- Supported: Search and Retrieval
- Supported: eISF Connectivity
- Supported: Real Time Collaboration
- Supported: TMF Health Check
- Supported: Inspection Readiness
- Supported: Milestone Tracking
- Supported: Unified with CTMS, EDC, and Safety & PV
Cloudbyz eTMF Screenshots
Cloudbyz eTMF Competitors
Cloudbyz eTMF Technical Details
| Deployment Types | Software as a Service (SaaS), Cloud, or Web-Based |
|---|---|
| Operating Systems | Unspecified |
| Mobile Application | Apple iOS, Android, Mobile Web |
| Supported Countries | Global |
| Supported Languages | Danish, German, English, Finnish, French, Italian, Japanese, Korean, Dutch, Portuguese, Russian, Spanish, Swedish, Thai, Chinese |